Sulfide-based catalysts for enantioselective epoxidation
The catalytic enantioselective synthesis of terminal epoxides is significantly more challenging than other epoxide classes. In particular, the asymmetric epoxidation of aldehydes via methylene transfer from sulfonium ylides is a process that has been traditionally bedevilled by low catalytic efficiency and poor product enantiomeric excess. We are attempting to circumvent these issues through mechanistic investigation and the design of new sulfides capable of promoting highly selective variants of this important reaction.
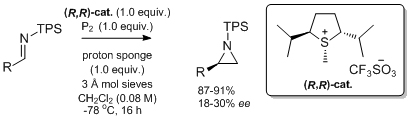
'The asymmetric synthesis of terminal aziridines by methylene transfer from sulfonium ylides to imines'
S. A. Kavanagh, A. Piccinini and S. J. Connon*, Org. Biomol. Chem. 2013, 11, 3535.
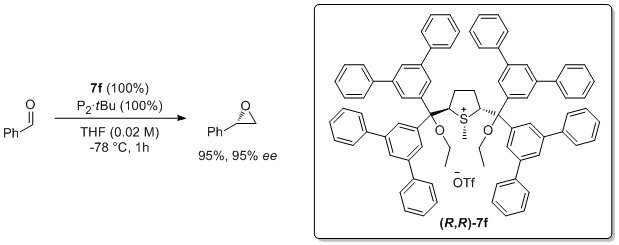
'Highly enantioselective ylide-mediated synthesis of terminal epoxides'
A. Piccinini, S. A. Kavanagh and S. J. Connon*, Chem. Commun. 2012, 48, 7814.
'Efficient catalytic Corey-Chaykovsky reactions involving ketone substrates'
S. A. Kavanagh, A. Piccinini and S. J. Connon*, Adv. Synth. Catal. 2010, 352, 2089.
Highlighted in Synfacts (2010, 1418)
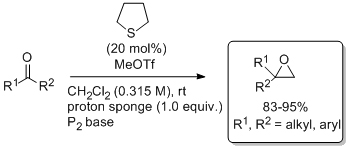
'Catalytic (asymmetric) methylene transfer to aldehydes'
A. Piccinini, S. A. Kavanagh, P. B. Connon and S. J. Connon*, Org. Lett. 2010, 12, 608.
Highlighted in Synfacts (2010, 349)
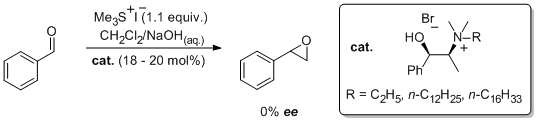
'N-Alkyl salts derived from ephedrine do not promote enantioselective Corey-Chaykovsky reactions involving sulfonium methylides under phase transfer conditions'
S. A. Kavanagh and S. J. Connon*, Tetrahedron Asymmetry 2008, 19, 1414.
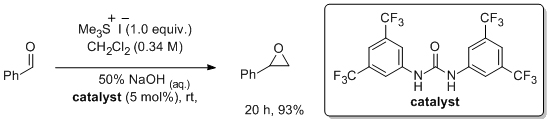
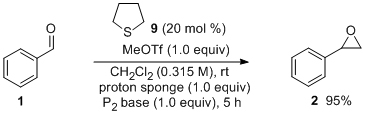
'Urea derivatives are highly active catalysts for the base-mediated generation of terminal epoxides from aldehydes and trimethylsulfonium iodide'
S. A. Kavanagh, A. Piccinini, E. M. Fleming and S. J. Connon*, Org. Biomol. Chem. 2008, 6, 1339.